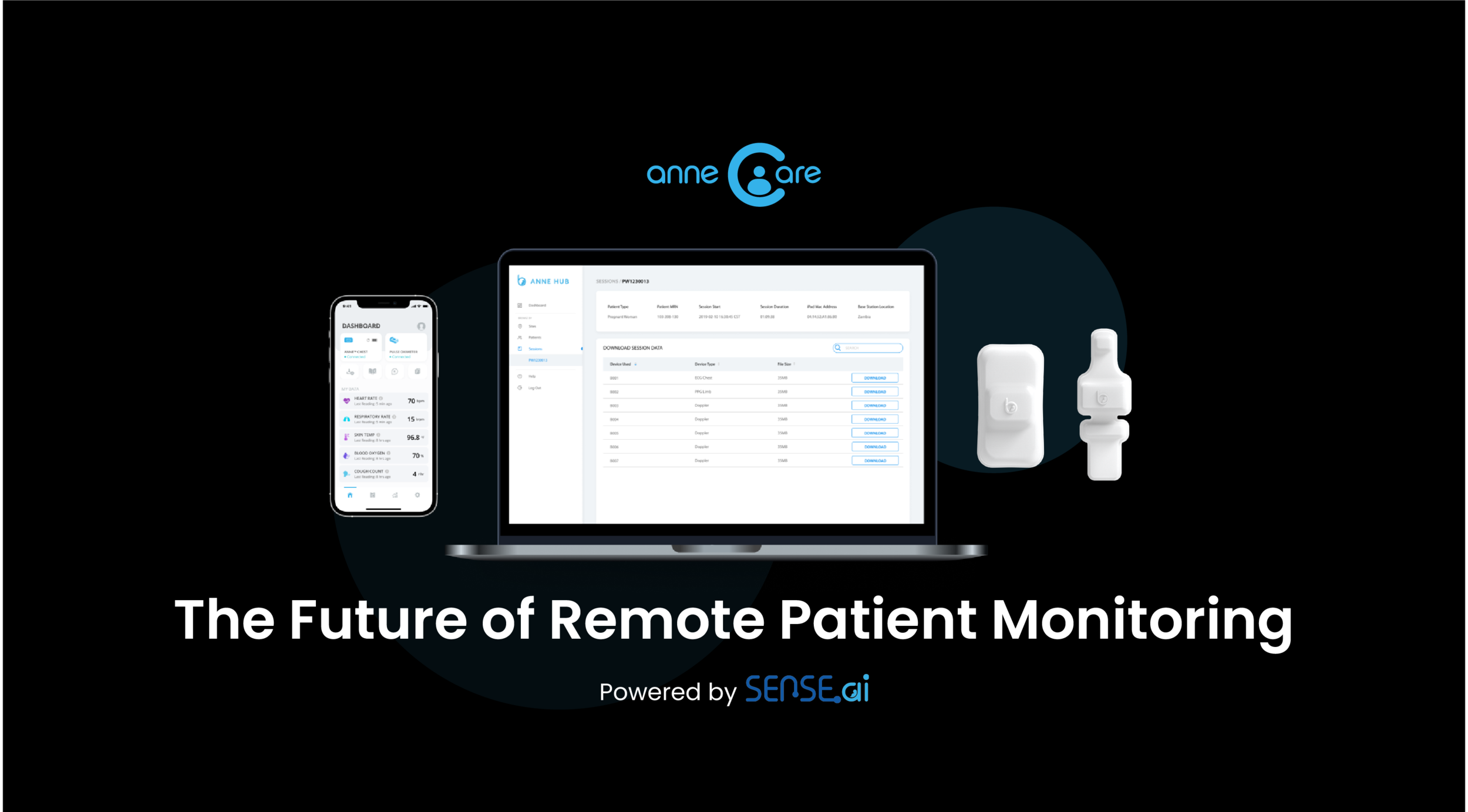


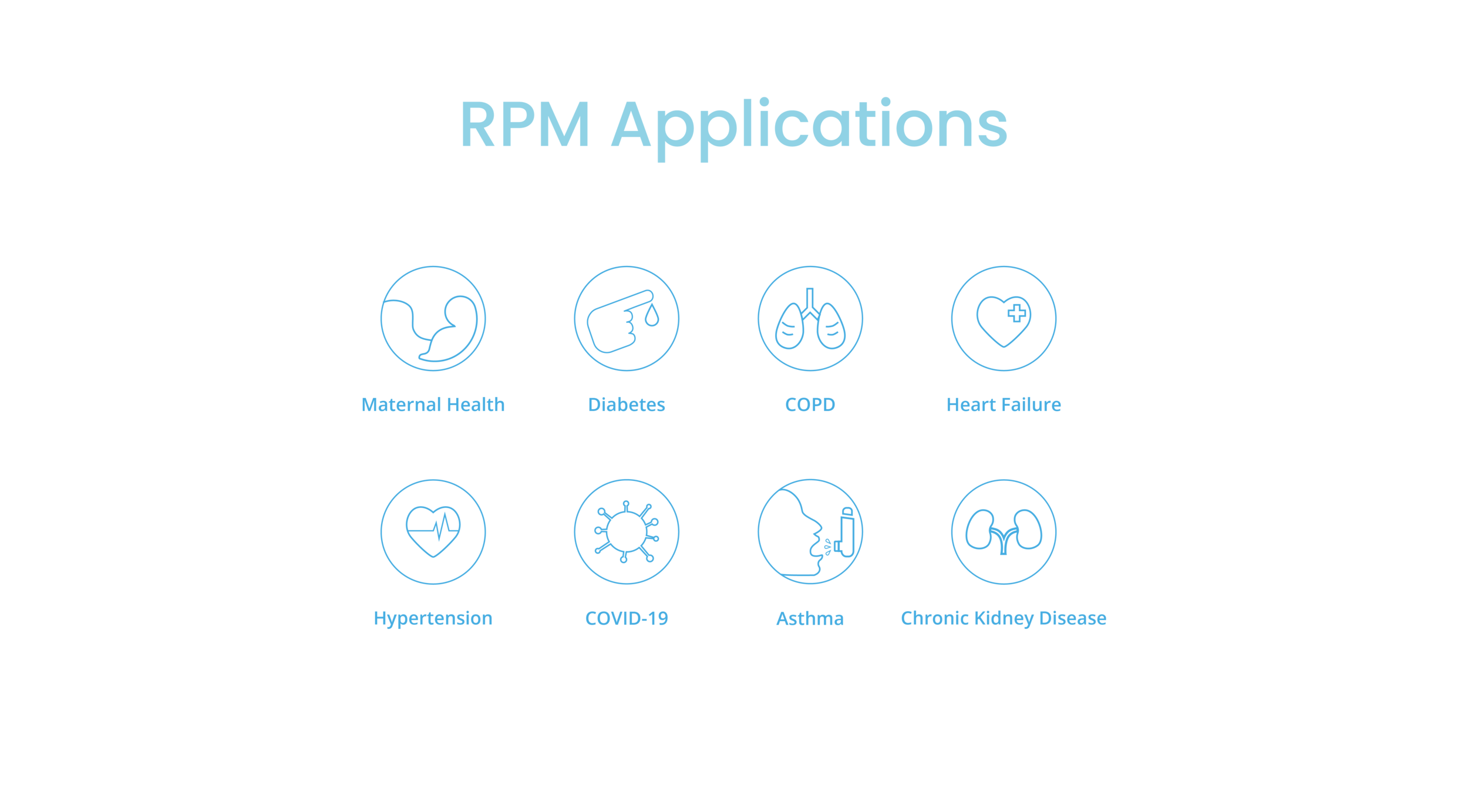
Notice to patients and providers, recommendations provided by ANNE Care are supportive and should not be solely or primarily relied upon to prevent, diagnose, or treat COVID-19 or co-existing conditions. ANNE Care is designed for both continuous, spot-checking, and trend monitoring. The ANNE Care system offers features not cleared by the FDA including home use, and integration of an existing FDA-cleared pulse oximeter. However, the FDA has issued a policy to help expand the availability and capability of non-invasive remote monitoring devices such as ANNE Care to facilitate patient monitoring while reducing patient and healthcare provider contact and exposure to COVID-19 for the duration of the COVID-19 public health emergency.
